
NEW! Accelerated TMS Treatments Now Available!
A New Breakthrough In Moderate To Severe Depression Treatment
This FDA-cleared treatment has been shown to effectively treat depression patients that have not responded sufficiently to traditional prescription medications.
(Click to watch the video)
Schedule A Free Consultation To See if TMS therapy Could Be right for you
Please complete the information below to be taken to our online calendar to schedule a free, no-obligation consultation to learn more about this treatment.
I understand that I may receive communications via email or text regarding this request.
Your information will be kept 100% confidential
FDA cleared
Covered under some insurance
Non-invasive, no anesthesia
- Does not affect cognitive function
- Excellent response and remission rate
- Outpatient procedure
FDA cleared
Covered under some insurance
Non-invasive, no anesthesia
Does not affect cognitive function
Excellent response and remission rate
Outpatient procedure
Schedule A No-Cost In-Office Consultation With One Of Our Specialists Today.
NOW AVAILABLE!
New Accelerated TMS!


At Invictus Clinic we're proud to offer the FDA Cleared Accelerated TMS therapy that reduces the typical 9-week treatment course down to just one week. This accelerated protocol means quicker relief from the debilitating symptoms of depression.
The Accelerated TMS protocol is based on the groundbreaking SAINT study (details below). This Stanford Accelerated Intelligent Neuromodulation Therapy saw incredible results with a staggering 90% remission rate among Major Depressive Disorder patients. Further evidence from the subsequent SNT study reaffirms this approach, demonstrating a robust remission rate of 78.6%.
Our confidence in this treatment is enhanced by the fact that we also use MagVenture systems TMS equipment - the same high-quality system employed by the Stanford research team in the SAINT study.
At Invictus Clinic, we aren't just offering therapy; we're offering a proven and non-invasive pathway to reclaiming your joy, your life, and your hope. Step into a brighter future with us today by scheduling your free, no-obligation consultation.
What Is TMS Therapy?
Transcranial Magnetic Stimulation, or TMS, is a drug-free solution for treating depression. It works by using magnetic pulses to stimulate certain areas of the brain that are underactive and causing depression. It's safe, effective, and doesn't involve any invasive procedures.
TMS = Transcranial Magnetic Stimulation
Series of pulsed magnetic waves to specific areas of the brain
FDA cleared for the treatment of major depressive disorder
Covered by some insurance providers
Safe, with high tolerability
Non-invasive treatment – no anesthesia, no injections, no medications
May be used with or without antidepressants (determined by physician)
Drug Free - no common antidepressant drug side effects
Does not affect cognitive function
Patients are able to resume daily activities right after treatment
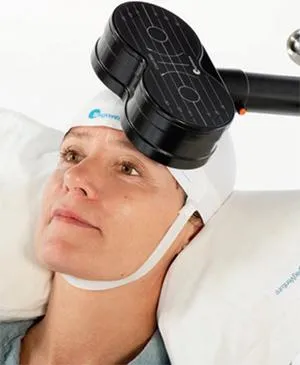
During treatment, magnetic pulses are delivered through a magnetic coil to stimulate nerve cells in the part of the brain controlling the mood.
Repeated stimulation of this area of the brain can have an antidepressant effect on people suffering from major depressive disorder (depression).

Schedule a free, no-obligation consultation in our online calendar to see if this treatment may be right for you.
During this consultation, you'll have the opportunity to get all of your questions answered to help decide if this might finally be the solution you've been looking for.

What Our Patients Say...*
Watch Anders' Story
Watch Charlie's Story
Watch Charlie's Story
Watch Cindy's Story
Watch Amy's Story
Watch Amy's Story
Are you or a loved one struggling with major depressive disorder?

Have you obtained no or inadequate relief from antidepressants, or is it difficult to tolerate the side effects?
Maybe TMS Therapy could be the solution?
Click the button below to schedule your free consultation today.
About Us






BRANDON GRINAGE, MD
Co-Owner, Medical Director
Brandon Grinage, MD is an Anesthesiologist practicing in northwest Georgia since 2012. He is a proud member of the American Society of Anesthesiologists and the Georgia Society of Anesthesiologists.
Dr. Grinage has seen first-hand the impact depression or PTSD can have on an individual and their family. He has seen the crippling effects of chronic aches and the lives destroyed by narcotic dependence and addiction. The potential that TMS Therapy has shown in numerous studies to help patients overcome depression, PTSD, chronic aches, and many other maladies is beyond significant. This treatment is inspiring and provides hope to the millions of Americans that have long felt overlooked, ignored, or beyond help. It is with this in mind that Dr. Grinage enthusiastically joined Wesley Karcher to open Invictus Clinic.

WESLEY KARCHER, MSN, CRNA
Co-Owner
Wesley Karcher has practiced as a Nurse Anesthetist (CRNA) since 2007. He is the President for the Georgia Association of Nurse Anesthetists (GANA) and an active member of the American Association of Nurse Anesthetists (AANA).
After years of working in multiple hospitals and medical practice settings, Wesley realized there was an unmet need for our patients with mental health and aches issues. As an anesthesia expert, he is well versed in all aspects of the administration of TMS Therapy. As a resident of Woodstock, GA, he is excited to offer this transformative care to better meet the mental health and aches treatment needs of residents in Cherokee County and many surrounding areas.
Wesley lives in Woodstock, GA with his wife and 3 children. Wesley loves coaching baseball for his son, traveling with his family, and is an avid backcountry hiker.

WESLEY KARCHER, MSN, CRNA
Co-Owner
Clinical Studies
Here are just a few of the 2,300+ research articles and scientific studies that have been done on Transcranial Magnetic Stimulation and published in the National Institue for Health's National Library of Medicine.

Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression (https://ajp.psychiatryonline.org/doi/10.1176/appi.ajp.2019.19070720)
“...Nineteen of 21 participants (90.5%) met remission criteria (defined as a score <11 on the Montgomery-Åsberg Depression Rating Scale). In the intent-to-treat analysis, 19 of 22 participants (86.4%) met remission criteria. Neuropsychological testing demonstrated no negative cognitive side effects.”
Accelerated repetitive transcranial magnetic stimulation (aTMS) for treatment-resistant depression (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3020591/)
“...Depression and anxiety significantly decreased following aTMS [Accelerated TMS] treatments, and improvements persisted 3 and 6 weeks later.”
Accelerated TMS for Depression: A Systematic Review and Meta-Analysis (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6582998/)
“...Overall, the meta-analysis suggested that aTMS [Accelerated TMS] improves depressive symptom severity.”
Accelerated repetitive transcranial magnetic stimulation in the treatment of depression (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5983543/)
“...It is feasible to provide accelerated rTMS treatment for outpatients with depression and this is likely to produce meaningful antidepressant effects.”
Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis (https://pubmed.ncbi.nlm.nih.gov/18447962/)
“...These findings show that high-frequency rTMS over the left DLPFC is superior to sham in the treatment of depression. The effect size is robust and comparable to at least a subset of commercially available antidepressant drug agents.”
Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials (https://pubmed.ncbi.nlm.nih.gov/23507264/)
“...HF-rTMS seems to be associated with clinically relevant antidepressant effects and with a benign tolerability profile.”

Stanford Neuromodulation Therapy (SNT): A Double-Blind Randomized Controlled Trial (https://ajp.psychiatryonline.org/doi/10.1176/appi.ajp.2021.20101429)
“...Overall, across the 4-week follow-up, 12 participants (85.7%) in the active SNT group met the criterion for response (a reduction ≥50% in MADRS score), and 11 (78.6%) met the remission criterion (a MADRS score ≤10) in at least one of the five post-treatment assessments.”
Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial (https://pubmed.ncbi.nlm.nih.gov/17573044/)
“...Transcranial magnetic stimulation was effective in treating major depression with minimal side effects reported. It offers clinicians a novel alternative for the treatment of this disorder.”
Accelerated rTMS: A Potential Treatment to Alleviate Refractory Depression (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6220029/)
“…our observations substantiate the potential of the accelerated stimulation to shorten the treatment duration from the depressed state to the response state. Any time gain from the depressed state to the recovered state is in the patients’ interest.”
Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial (https://pubmed.ncbi.nlm.nih.gov/20439832/)
“...Daily left prefrontal rTMS as monotherapy produced statistically significant and clinically meaningful antidepressant therapeutic effects greater than sham.”
High-frequency repetitive transcranial magnetic stimulation accelerates and enhances the clinical response to antidepressants in major depression: a meta-analysis of randomized, double-blind, and sham-controlled trials (https://pubmed.ncbi.nlm.nih.gov/23473357/)
“...HF-rTMS is a promising strategy for accelerating clinical response to antidepressants in major depression, providing clinically meaningful benefits that are comparable to those of other agents such as triiodothyronine and pindolol. Furthermore, HF-rTMS seems to be an acceptable treatment for depressed subjects.”
Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression (https://pubmed.ncbi.nlm.nih.gov/28541649/)
“...Multiple randomized controlled trials and published literature have supported the safety and efficacy of rTMS antidepressant therapy.”

Our Location
203 Woodpark Place
Building B (1st Floor)
Woodstock, GA 30188
* The testimonials presented on this site are individual experiences, reflecting real life experiences of those who have received the treatment presented here. However, they are individual results and results do vary. While the vast majority of our patients do experience reduced pain and improved physical condition from this treatment, we do not claim that every patient will experience these improved conditions. Additionally, these testimonials are not intended to make claims that these products can be used to diagnose, treat, cure, mitigate or prevent any disease. These claims have not been evaluated by the FDA.
The information contained on this page has not been evaluated by the FDA and is not intended to diagnose, treat or cure any disease, injury or illness.
Copyright 2023 - All Rights Reserved